Click on image to enlarge
Many cells in the body produce nitric oxide; however, its production by the vascular endothelium is particularly important in the regulation of blood flow. Abnormal production of nitric oxide, as occurs in different disease states, can adversely affect blood flow and other vascular functions. Nitric oxide is one of the few gaseous signalling molecules known and is additionally exceptional due to the fact that it is a radical gas. It is a key vertebrate biological messenger, playing a role in biological processes.
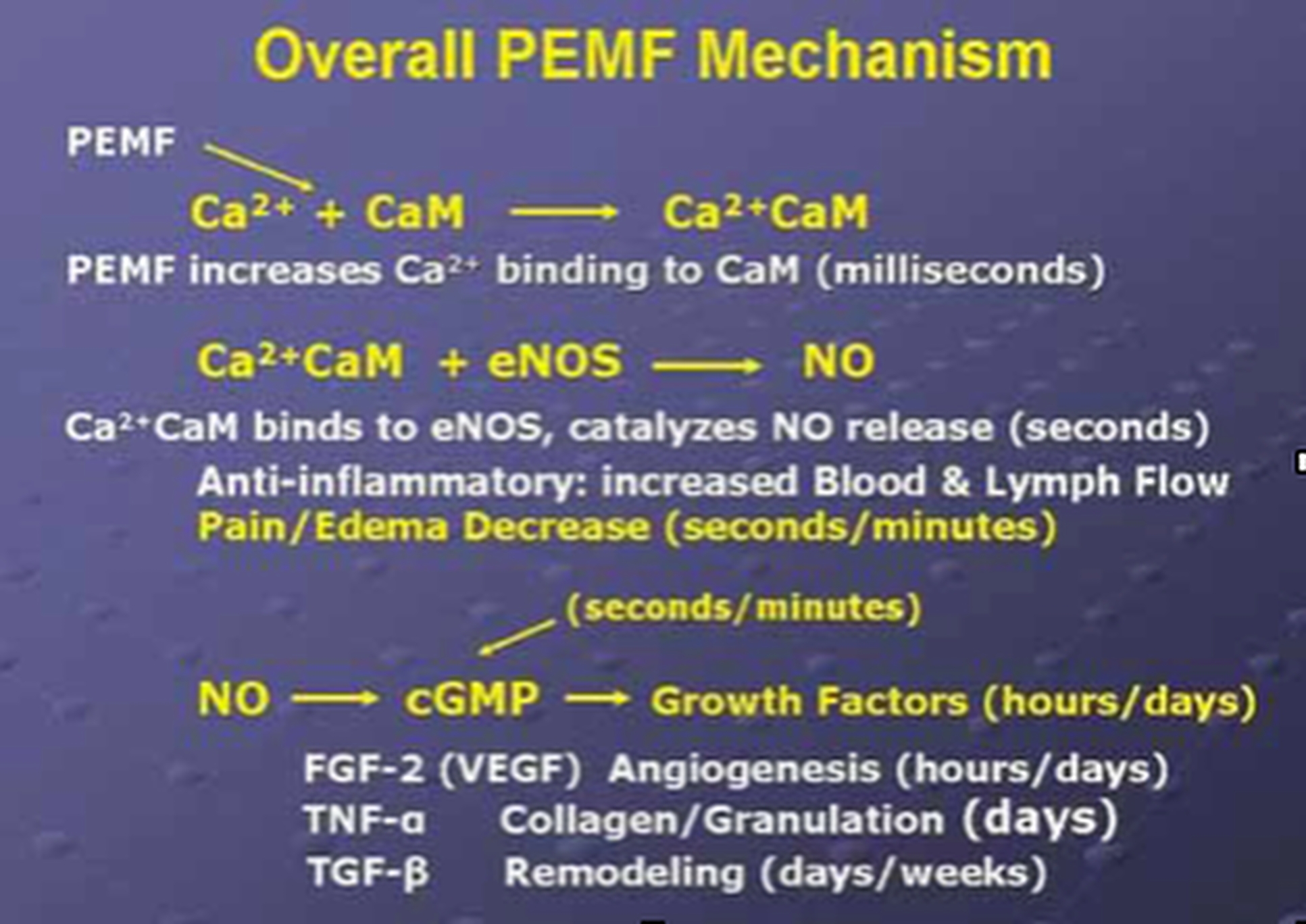
The March/April 2009 Aesthetic Surgery Journal published a study: “Evidence-Based Use of Pulsed Electromagnetic Field Therapy in Clinical Plastic Surgery” that summarizes the evolution in the understanding of the physiological effects of PEMF therapy on cells and tissues. Studies emerged suggesting that PEMF could modulate the production of growth factors and began to focus on enzyme systems with well-characterized calcium (Ca2+) dependence. By the mid-1990's, researchers were investigating the effects of electrical and PEMF signalling on intracellular Ca2+, specifically the binding of Ca2+ to calmodulin (CaM), using the knowledge that CaM dependent cascades were involved in tissue repair. The most recent studies of the PEMF transduction pathway have concentrated upon the Ca/CaM-dependent nitric oxide cascades, the growth factor cascades involved in tissue healing. It is within this system that the effectiveness of PEMF is now understood to function. PEMFs modulate the calcium binding kinetics to calmodulin. Calcium/calmodulin (Ca/CaM) then activates nitric oxide synthase (NOS) in several different isoforms. When injury occurs, large amounts of nitric oxide are produced by long-lived inducible nitric oxide synthase (iNOS). In this cascade, tissue levels of nitric oxide persist, and the prolonged presence of this free radical is pro-inflammatory, which accounts for the leaky blood vessels associated with pain and swelling. In contrast, the endothelial and neuronal nitric oxide synthase isoforms (respectively eNOS and nNOS) produce nitric oxide in short bursts that can immediately relax blood and lymph vessels. These short bursts of nitric oxide also lead to the production of cyclic guanosine monophosphate (cGMP), which in turn drives growth factor production. Interestingly, iNOS is not dependent on CaM, while the constitutive or cNOS (eNOS or nNOS) cascade is dependent on the binding of Ca/CaM.
Therapies that could accelerate Ca/CaM binding, therefore, should impact all phases of tissue repair, from initial pain and swelling to blood vessel growth, tissue regeneration, and remodeling. As shown in the diagram to the left, this mechanism has been proposed as a working model for PEMF therapeutics.
Nitric oxide, known as the endothelium-derived relaxing factor or EDRF, is biosynthesized endogenously from L-arginine, oxygen and NADPH by various nitric oxide synthase (NOS) enzymes. Dr. Richard E. Klabunde explains the synthesis of nitric oxide from the amino acid L-arginine by the enzymatic action of nitric oxide synthase (NOS). There are two endothelial forms of NOS: constitutive NOS (cNOS; type III) and inducible NOS (iNOS; type II). In addition to endothelial NOS, there is a neural NOS (nNOS; type I) that serves as a transmitter in the brain and in different nerves of the peripheral nervous system, such as non-adrenergic, non-cholinergic (NANC) autonomic nerves that innervate penile erectile tissues and other specialized tissues in the body to produce vasodilation.
Click on image to enlarge
The endothelium (inner lining) of blood vessels uses nitric oxide to signal the surrounding smooth muscle to relax, thus resulting in vasodilation and increasing blood flow. Under normal conditions, nitric oxide is continually being produced by cNOS in the blood vessels. The activity of cNOS is Ca/CaM-dependent and produces vascular relaxation when the endothelium is intact. The activation of the other isoform of endothelial NOS is iNOS and is not calcium-dependent. Under normal conditions, the activity of iNOS is very low. The activity of iNOS is stimulated during inflammation by bacterial endotoxins or cytokines such as tumor necrosis factor (TNF) and interleukins. During inflammation, the amount of nitric oxide produced by iNOS may be a 1,000-fold greater than that produced by cNOS.
Copyright 2016 Magnus Magnetica, LLC / Unauthorized use or duplication is prohibited by law.